Electronic and paper CRF design in clinical trials
The UKTMN advises sponsors and investigators make paper medical records and data collection forms attractive and as easy as possible to complete. eClinical facilitates CRF design in clinical trials that produces quality data for analysis – meeting study protocol as well as ICH guidelines for case report forms.
CRF design in clinical trials for ease of completion and use
It is good practice to have a clear identity for the trial and design data capture forms that clearly states the activity and sponsor branding.
You can start with a blank page and design a paper survey, form or questionnaire using the intuitive drag-and-drop CRF designer, adding fields and labels as you go.
Design elements, such as logos, borders, headers and font styles can all be added to make the form follow your trial or sponsor branding. If you prefer, you can scan in an existing data capture form and overlay the structure on top to allow the system to capture your existing designs as well as new forms.
Many of your data collection requirements need to be configured at this stage. Settings for numeric or date ranges, mandatory fields and double keying can be defined on a per field basis, allowing for truly robust results.
How you configure this early stage is key to trial outcomes as it will provide the basis of your information gathering, so take the time to get CRF design in clinical trials right. ePC can offer our extensive experience to help you create scannable forms at this (and any other) stage, depending on the level of support you require.
Electronic data capture (EDC)
With the optional eForm module, eClinical can be used as a hybrid paper and electronic data capture (EDC) system.
eClinical allows paper CRF’s to be exported from the CRF Designer as dynamic PDF or HTML forms for end-users to complete on-screen and submit electronically back to the verification module.
We also provide stand-alone EDC systems that simplify compliance with ICH GCP E6 R2 standards and other regulations such as 21CFR Part 11 with online forms, web interfaces, audit trails, access control, version tracking, electronic signatures and more.
Typical form components

Choice fields
Set up simple tick boxes or multiple option tick boxes in a variety of styles.
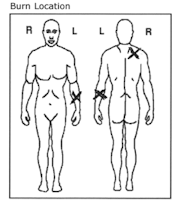
Image zones
Capture images, diagrams or data from multi-line machine print labels.

Signature zones
To confirm if a paper CRF has been authorised with a signature.
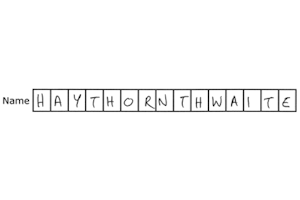
Constrained print fields
eClinical reads handwriting most effectively when it is laid out in a constrained print field, with one character per box.

Capture zones
For quickly capturing more complex fields such as “Other Comments:” by either predictive typing, coding or manual entry.

Barcodes
Many industry standard bar codes can be read from scanned images including two dimensional bar codes such as PDF417.
Paper CRF/questionnaires library

Avon Longitudinal Study of Parents and Children (ALSPAC)






North Cumbria University NHS Hospitals Trust



London School of Hygiene and Tropical Medicine / MenAfricar

London School of Hygiene and Tropical Medicine / MenAfricar

If you want to learn more about designing data capture forms and CRF development, or wish to book a demonstration, please contact us.
How to optimise CRF’s and other trial forms for scanning

Choice fields
Add a choice field to your form to accept a single choice (e.g. yes/no) or multiple choice (e.g. select option A/B/C/D).
Choice Fields consist of a single column, or row, of choices and offer an array of different styles including bubbles, boxes, underlines, responses and brackets.
To improve OMR (Optical Mark Recognition) accuracy, it is recommended to use bubbles and boxes rathar than circling a response or marking within brackets.
Cornerstones
Cornerstones will assist with page alignment, de-skewing images and adjusting for print or scan size variations.
Cornerstones
Cornerstones will assist with page alignment, de-skewing images and adjusting for print or scan size variations.
Constrained print fields
Handwriting is most effectively read by ICR (Intelligent Character Recognition) technology when it is laid out in a constrained print field, with one character per box.
Form designers can add a constrained print field to gather data such as names, dates, and numeric figures. The boxes act as guides for the person filling out the form, with one dedicated space for each letter, number or character in the response.
Capture Zones
Capture Zones are typically used to capture blocks of cursive handwriting or other unquantifiable data. They are presented to the operator to allow high-speed coding or re-keying of the content. Advanced features, such as mark identification or predictive typing, help speed up this process.
Cornerstones
Cornerstones will assist with page alignment, de-skewing images and adjusting for print or scan size variations.
Cornerstones
Cornerstones will assist with page alignment, de-skewing images and adjusting for print or scan size variations.
Image zones
Image zones can capture machine-print text, barcodes and pictures. This element is often used to capture printed address labels, barcodes or provide images of handwritten comments.
Form identification block
Barcode
Unconstrained Likert scales
An unconstrained Likert scale (named after Rensis Likert) can be used to allow respondents to provide a rating or response without subconscious bias caused by limiting ratings to pre-defined values within a range.
If you want to learn more about hybrid paper and electronic data capture in clinical trials, or wish to book a demonstration, please contact us.